FLEXcyte 96
In the field of pre-clinical drug development, assessing drug-induced effects on cardiac contraction stands as a pivotal phase, ensuring the reliability of data crucial for subsequent clinical studies. Departments focused on safety, toxicology, and efficacy worldwide are in search of a contemporary contractility assay that unites the trusted methodology of the Langendorff Heart with the efficiency and predictive nature of modern high-throughput human cell models.
Our joint forces with Nanion Technologies resulted in a groundbreaking development: the FLEXcyte Technology. This innovation represents a paradigm shift, offering a more streamlined and predictive approach to conducting cardiotoxicity studies on high throughput level, marking a leap towards enhanced efficiency and reliability in drug evaluation.
As members of the HESI Cardiac Safety Technical Committee, we are integral contributors to an association dedicated to mitigating drug-related cardiovascular side effects. Here, the evaluation of chronic cardiac risk is performed with the FLEXcyte technology as part of the Hesi Stem Cell Working Group.
FLExcyte 96 Parameters
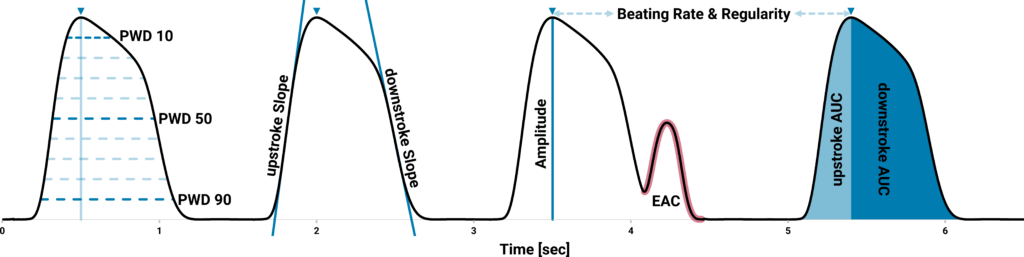
A specialized software enables comprehensive analysis of essential contractile parameters. By utilizing an adaptive signal detection algorithm, positions and values of beating events, rising and falling times, beat durations, arrhythmia and most importantly contraction force (mN/mm2) are extracted.
This cutting-edge technology facilitates recordings within an environment that closely mimics in vivo conditions, replicating the mechanical conditions of the heart. It serves as a significant advancement in the evaluation of drug candidates in studies pertaining to cardiac safety, toxicity, and efficacy.
Label-Free Contractility Analysis
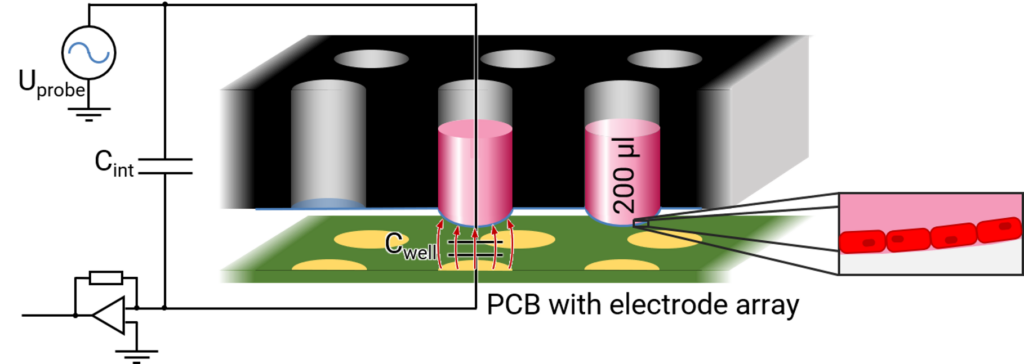
The FLEXcyte technology is based on a specialized FLEXcyte-96 (FLX-96) well plate, a pioneering 96-well platform featuring high-precision, ultra-thin, and hyperelastic silicone membranes. Unlike traditional stiff plastic surfaces, these membranes serve as the foundation for human iPSC-derived cardiomyocytes and other contractile cell types.
Integrated into the FLEXcyte 96 device, an independent benchtop incubation system, these unique FLX-96 plates host cells forming monolayers on flexible substrates. The contraction of the synchronized cell layers is meticulously recorded. While being deflected by the weight of the culture medium, rhythmic contraction of the cells lift the membranes in the 96-well upwards. By measuring the changes in deflection, the mechanical stress is calculated in a label-free manner.
Optical Pacing
Optical stimulation is an important tool for fostering cardiomyocyte maturation in vitro and effectively regulating cardiac rhythms when necessary.
In this process, human iPSC-derived cardiomyocytes are cultivated on FLEXcyte 96 plates and transfected with channelrhodopsin2 using the highly efficient Fuse-It-mRNA transfection kit by beniag GmbH. Controlled, even stimulation of cells is achieved through the CardioExcyte 96 SOL’s optical lid, equipped with an individual LED for each well. Stimulation sweeps, lasting at least 30 seconds, or longer if required, can be seamlessly conducted.
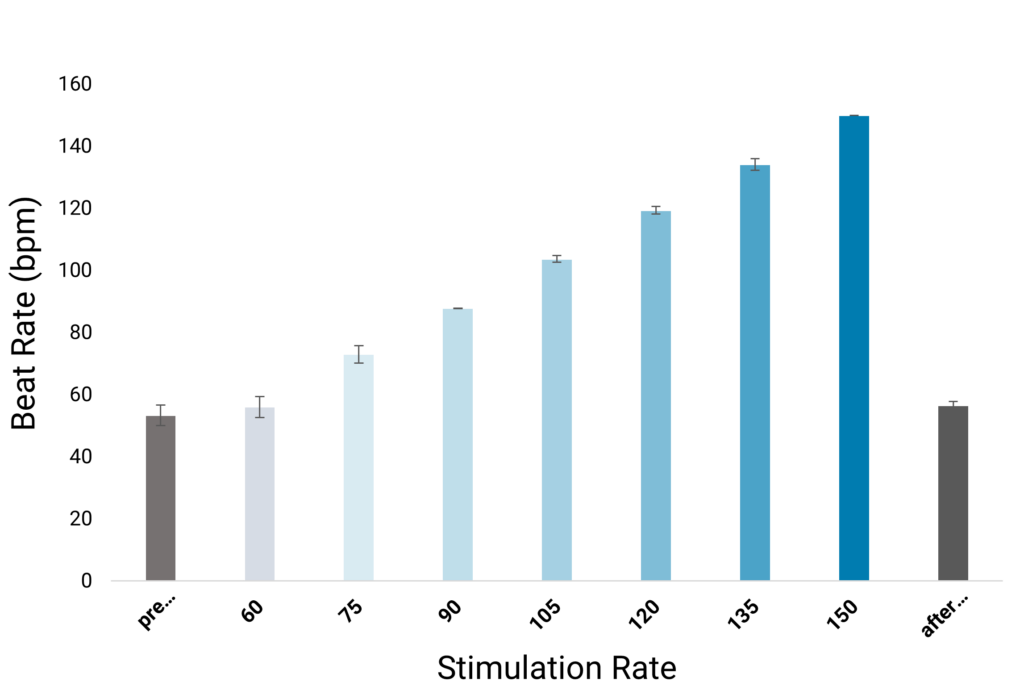
Mechanical Pacing
Utilizing controlled mechanical stimulation is crucial for promoting cardiomyocyte maturation and efficiently managing cardiac rhythms as needed.
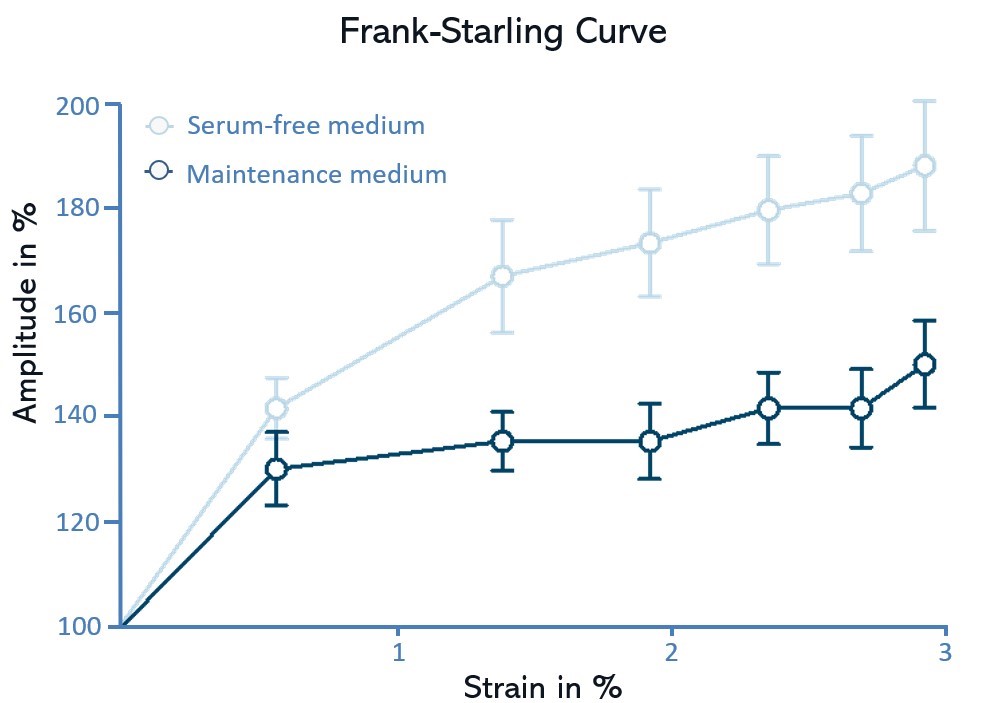
A static, dynamic, or ramp approach is feasible to address various cardiac-related concerns, including hypertension, maturation, and replication of the Frank-Starling mechanism.
In this process, human iPSC-derived cardiomyocytes are cultivated on FLEXcyte 96 plates. Pneumatic pressure is applied by the FLEXcyte 96 system, allowing controlled, even stimulation of cells. Stimulation sweeps, lasting at least 30 seconds, or longer if required, can be seamlessly conducted.
Case Study 1: Cell Maturation Effect
Conventional cultivation methods rely on rigid glass or plastic surfaces, which fail to emulate the natural physiological conditions experienced by cells in the human body. In contrast, FLEXcyte plates recreate the mechanical environment akin to real biological tissue by incorporating ultra-thin hyperelastic silicone membranes. These membranes create a biohybrid with the tissue applied, closely mimicking the conditions found in tissue in vivo.
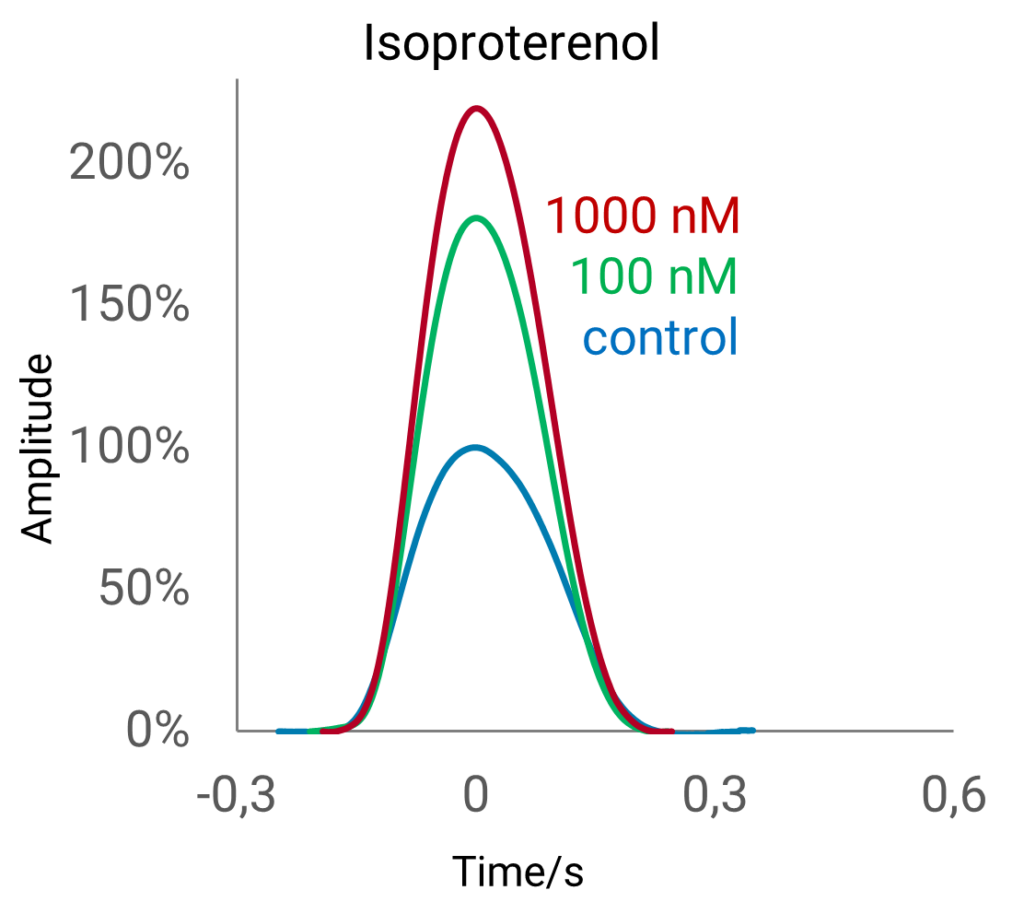
This natural environment fascilitates the development of a mature phenotype of cultured human iPSC-derived cardiomyocytes in vitro. The pro-maturation effect induced by the FLEXcyte 96 technology sets it apart from other contractility assays commonly employed in drug development, offering an important feature that is unmatched by conventional methods. Cell maturation can be effectively analyzed at a pharmacological level by employing compounds known for their positive inotropic effects, such as isoproterenol (ISO).
Case Study 2: Chronic Cardiotoxicity Evaluation
In cancer research, the intense development of targeted therapeutics such as tyrosine kinase inhibitors (TKIs) has brought tremendous improvement to the survival rate of cancer patients, compared to traditional anti-cancer treatments like anthracyclines. Nevertheless, both therapeutics, TKIs and anthracyclines, still lead to adverse cardiotoxic side effects such as left ventricular dysfunction and heart failure.
Here, the applicability of hiPSC-CM contractility measurements for chronic toxicological assessment is shown. 15 kinase inhibitors and 3 anthracyclines with well-known cardiotoxic profiles are selected to evaluate the reproducibility of clinical data. The results show the expected cardiotoxic effects, including negative inotropy, induction of proarrhythmic events and ceased beating in a dose- and time-dependent manner, proving the advantage of the FLEXcyte system over commonly used cell-based assays for chronic preclinical cardiac risk assessment.