CardioExcyte 96
Understanding compound- or chemically-induced cellular changes holds paramount importance for the development of new therapeutics or chemicals. A continuous and label-free monitoring of cellular activity serves as a pivotal metric, offering insights into both acute and chronic cellular changes. This impedance-based approach ensures that no subtleties in cytotoxic responses are overlooked, encompassing diverse cell types — non-contractile, such as hepatic or cancer cells, alongside contractile cardiac cells.
The impedance-based CardioExcyte 96 is a cutting-edge screening technology, designed specifically for the comprehensive evaluation of cellular morphology and electrophysiological alterations. By utilizing an electric current impeded by the cell monolayer, this technology enables real-time analysis of both acute and chronic cell activity. In addition, this technology fascilitates the analysis of electric field potential recordings, providing additional insights that supplement traditional patch clamp electrophysiology.
This CiPA (Comprehensive in vitro Proarrhythmia Assay) applied service approach revolutionizes the landscape of compound screening and analysis, offering a holistic understanding of cellular responses and electrical dynamics with unparalleled efficiency.
Label-Free Morphological Analysis
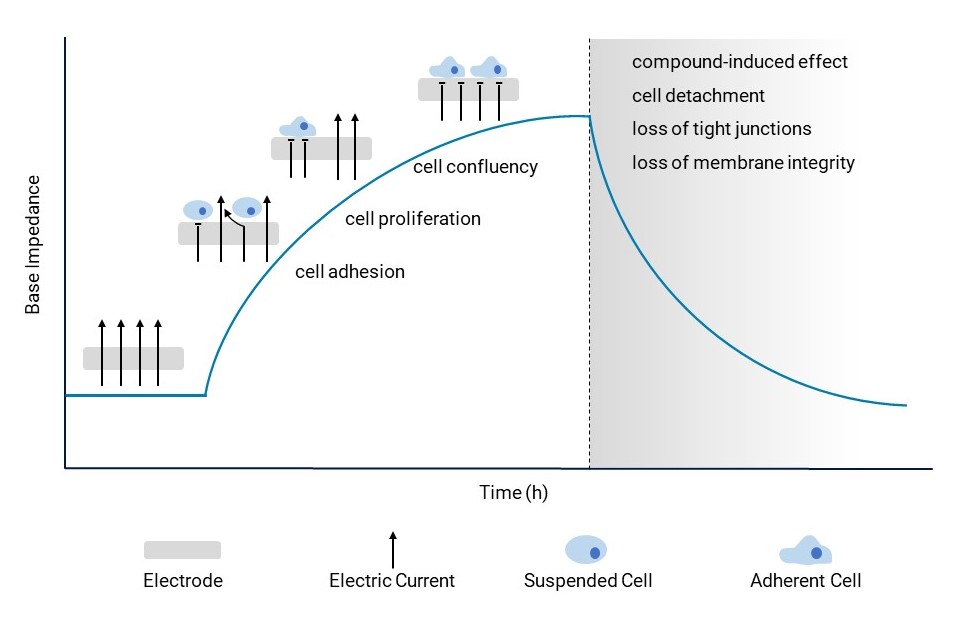
The base impedance parameter serves as a crucial tool for examining cellular changes. Cell adhesion, proliferation and confluency induce an increase in base impedance (cell signal). When compounds trigger cell detachment or disrupt tight junctions or membrane integrity, it results in a discernible shift in base impedance. This shift signals potential hazardous side effects on cellular morphology, offering a window into the compound’s impact.
Electrical and Optical Stimulation
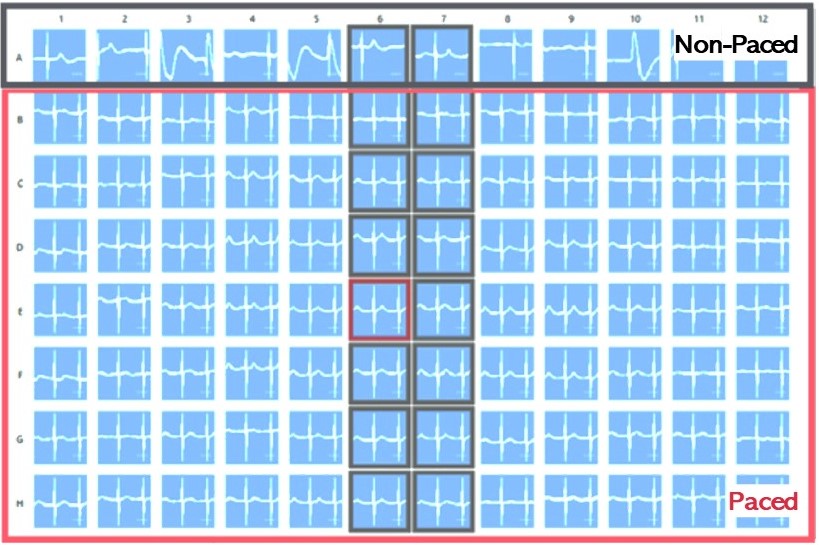
Pacing of cardiomyocytes is an important tool for effectively regulating cardiac rhythms when necessary and fostering cardiomyocyte maturation in vitro.
In this process, human iPSC-derived cardiomyocytes are cultivated on special CardioExcyte (NSP) plates and either transfected with channelrhodopsin2 (ChR2) using Fuse-It-mRNA transfection kit by beniag GmbH for optical stimulation or electrically paced via a stimulation electrode. Controlled stimulation sweeps, lasting at least 30 seconds, or longer if required, can be seamlessly conducted.
Case Study 1: Monitoring Compound-Induced Cellular Toxicity
Anthracycline-based cancer therapies, while effective against various solid and hematopoietic cancers, have been associated with triggering cardiovascular complications. Doxorubicin stands out for its exceptional efficacy, but its clinical utility is limited due to the significant risk it poses for its cardiotoxic side effect. Doxorubicin-treated hiPSC-derived cardiomyocytes monitored chronically, show a decrease in base impedance as metric for cardiotoxicity.
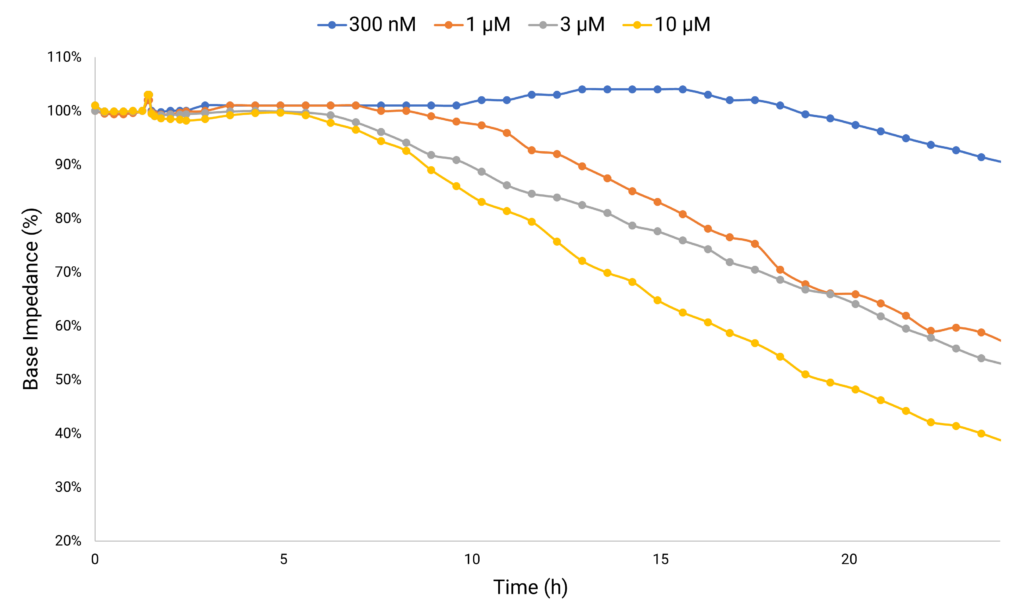
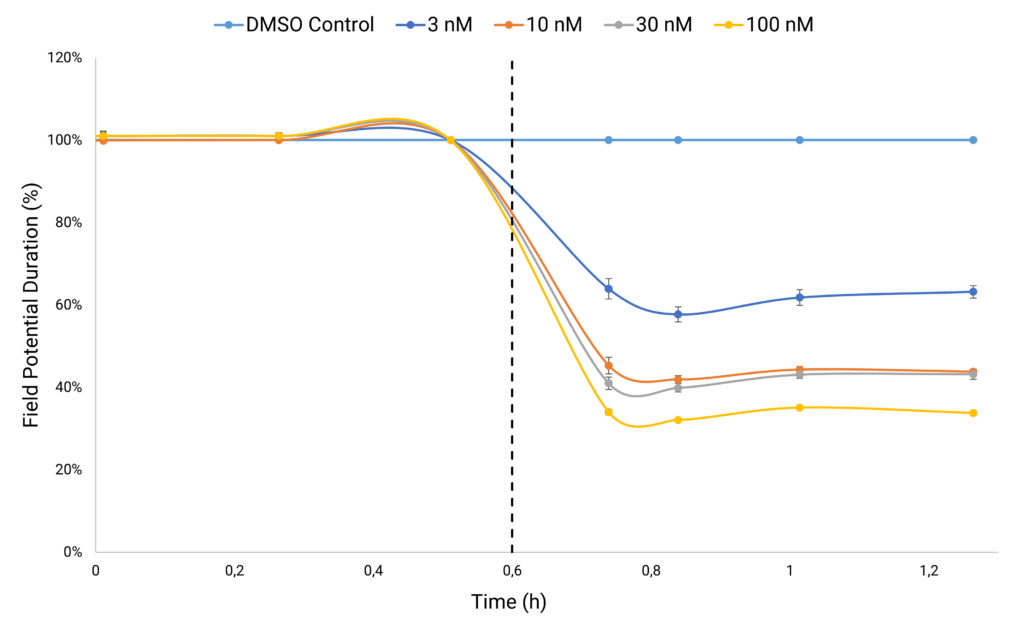
Case Study 2: Recording Electrophysiological Changes
Simultaneously, within the same wells, Electric Field Potential (EFP) recordings enable the analysis of MEA-like electrophysiological properties. Monitoring EFP raw traces and analyzing field potential durations offer critical insights into alterations in cell electrophysiology pre and post compound treatment. Analyzing the electrophysiological impact of L-type calcium channel blocker nifedipine on human iPSC-derived cardiomyocytes reveals a concentration-dependent decrease in Field Potential Duration. This reduction serves as a metric indicating a decrease in intracellular calcium levels.